About Us
LambdaGen Therapeutics is based on a proprietary genome engineering tool called LIGIT (Lambda (λ)-Integrase Genome Insertion Tool) that allows specific insertion of large multi-gene cassettes or DNA fragments at safe harbour sites in human genome, empowering us to propel our research and development efforts to develop innovative solutions in the field of cell and gene therapy.
Our Technology
LIGIT (Lambda-Integrase Genome Insertion Tool)
LIGIT is a non-viral technological advance in the form of a novel λ-integrase mediated site-specific recombination system that requires
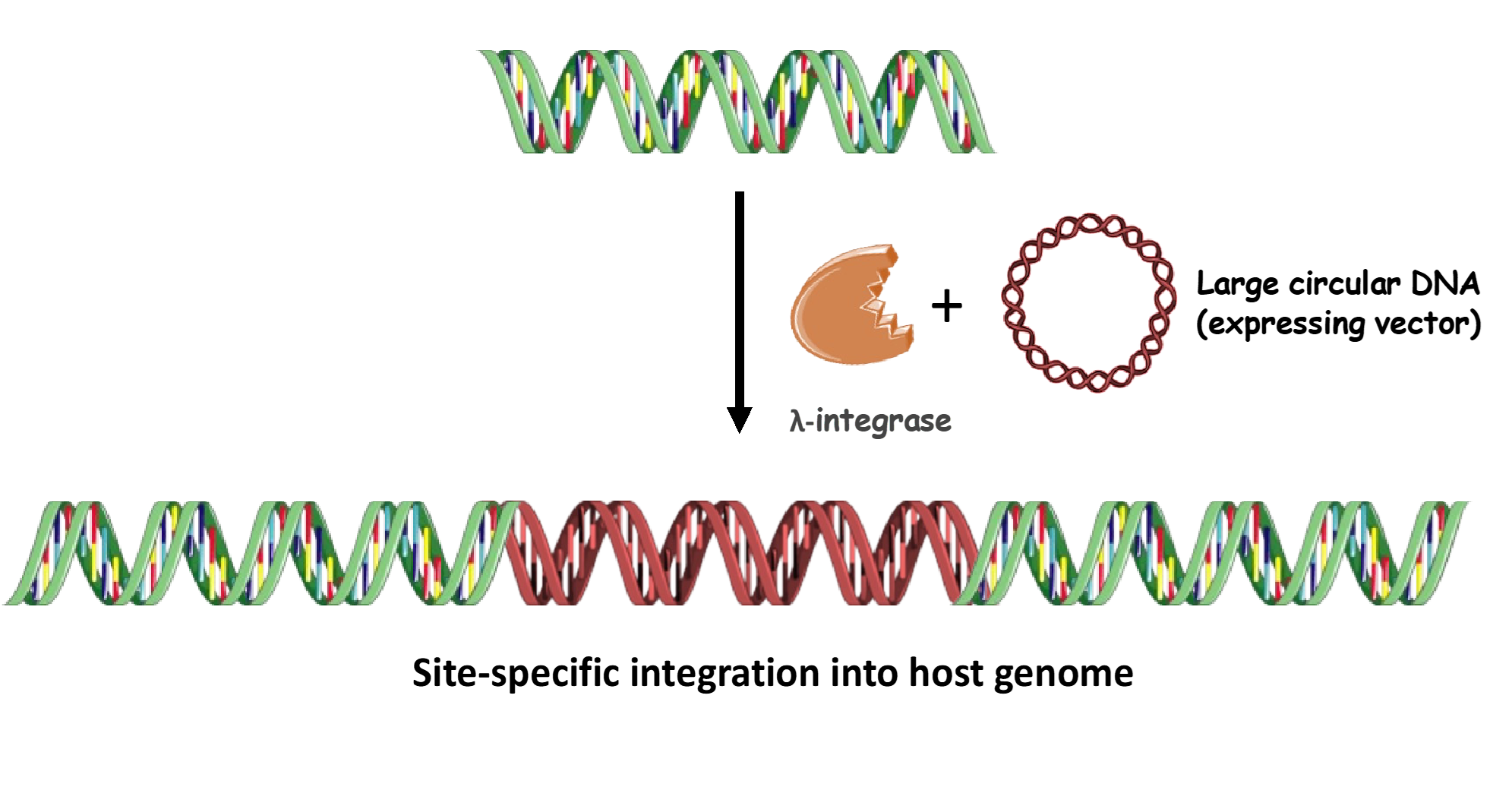
How does it work?
Our Platforms based on LIGIT
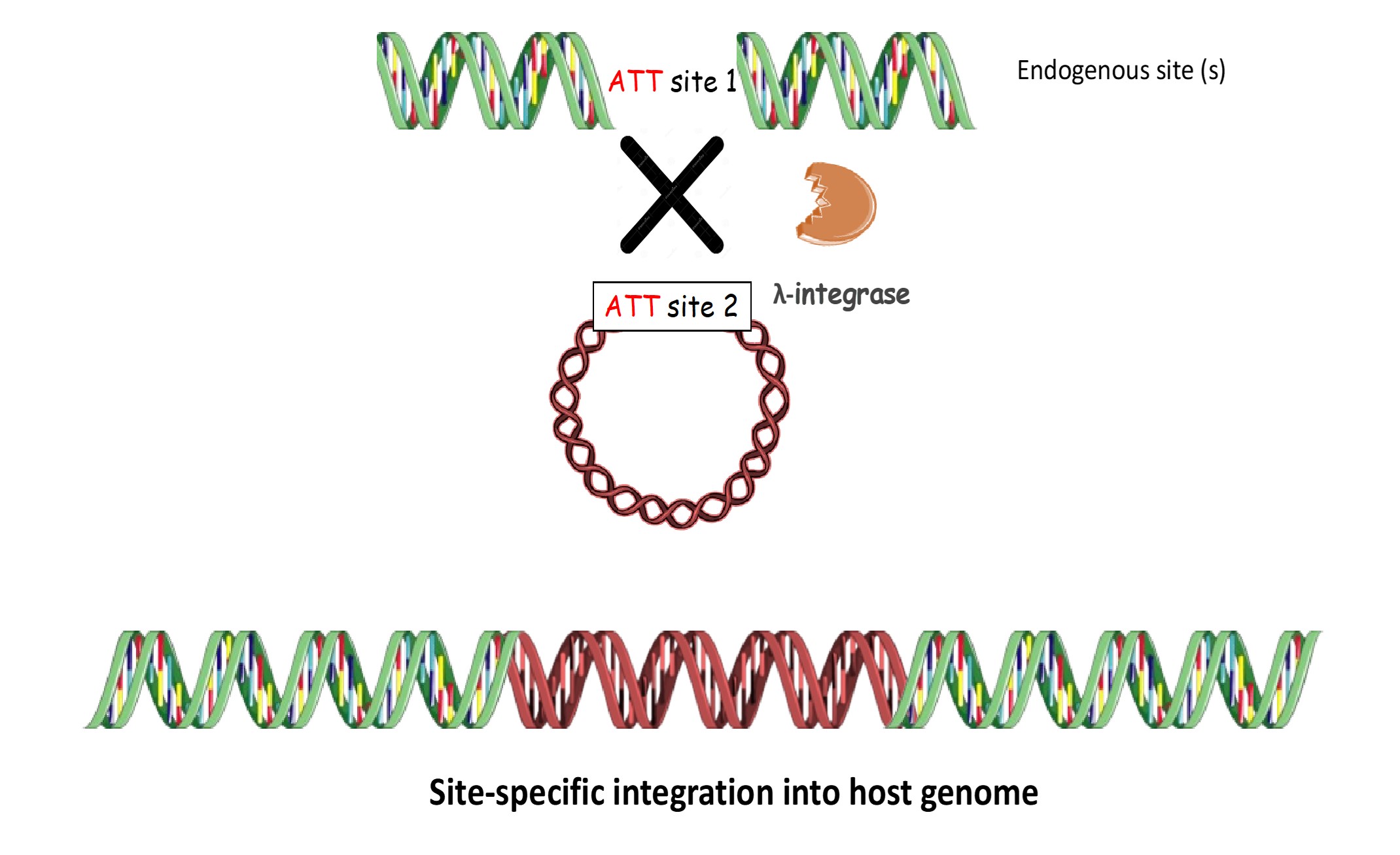
PLATFORM 1: Targeting specific endogenous site (s) in human genome
This involves targeting of a specific endogenous site (s) naturally present in human genome, termed as att(H) (att site 1) with the target vector comprising of attL/P (att site 2) using λ-integrase.
This has been validated in various cell lines including-
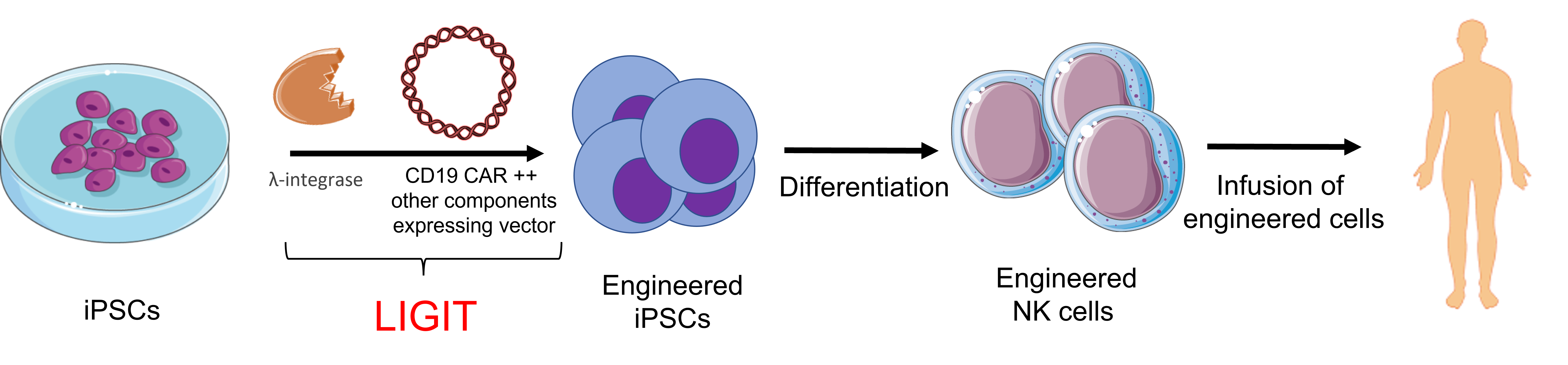
Current focus - Engineering and manufacture of iPSC derived NK cells for cancer immunotherapy
LambdaGen aim to develop an off-the-shelf and affordable multifunctional CAR-NK (m-CAR-NK) cells with functionally enhanced anti-tumor characteristics against B-cell lymphoma. Our strategy includes safe and seamless transgenesis of large DNA payloads with advanced Chimeric Antigen Receptors (CAR) designs including multiple antigen targets, key proliferation interleukins, killing signals into the genome of iPSCs to be differentiated into NK cells.
This would create a renewable source of iPSC derived m-CAR-NK cells, which will provide simultaneous biological improvements- a) enhanced tumor specificity; b) avoid tumour immune escape; c) sustained proliferative signals; d) enhanced tumoricidal activity; e) unlimited safe source of m-CAR-NK cells.
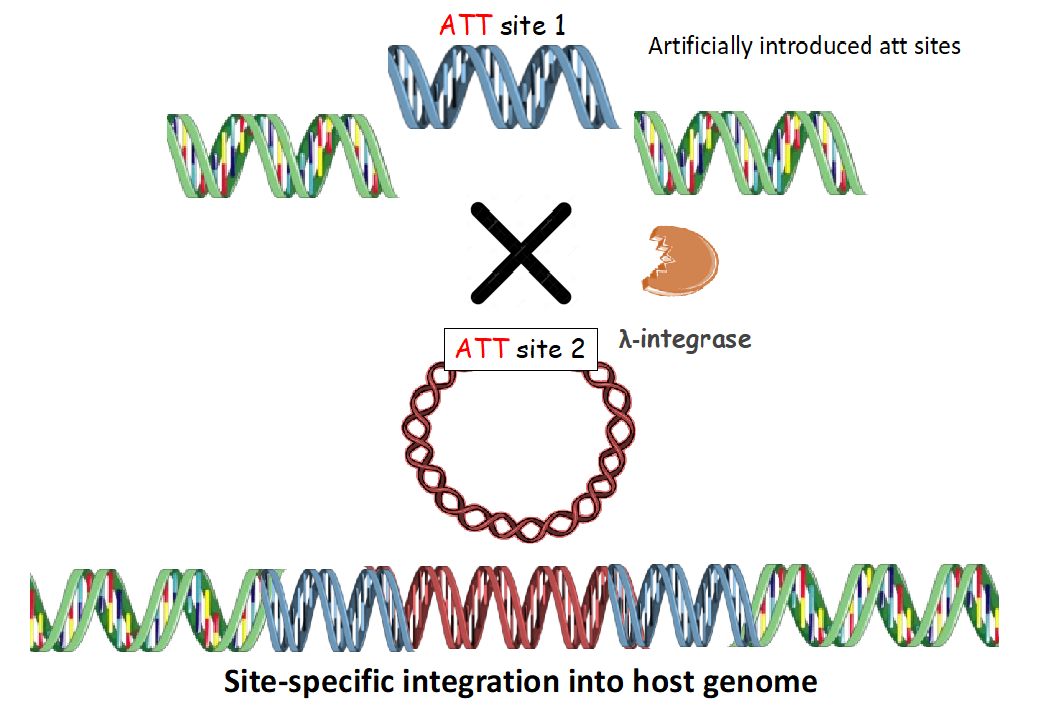
PLATFORM 2: Targeting artificial introduced landing pad site (s) in human genome
This involves targeting of artificially introduced site (s) in host genome, termed as att(L/P) (att site 1) with the target vector comprising of attH (att site 2) using λ-integrase. This has been validated.
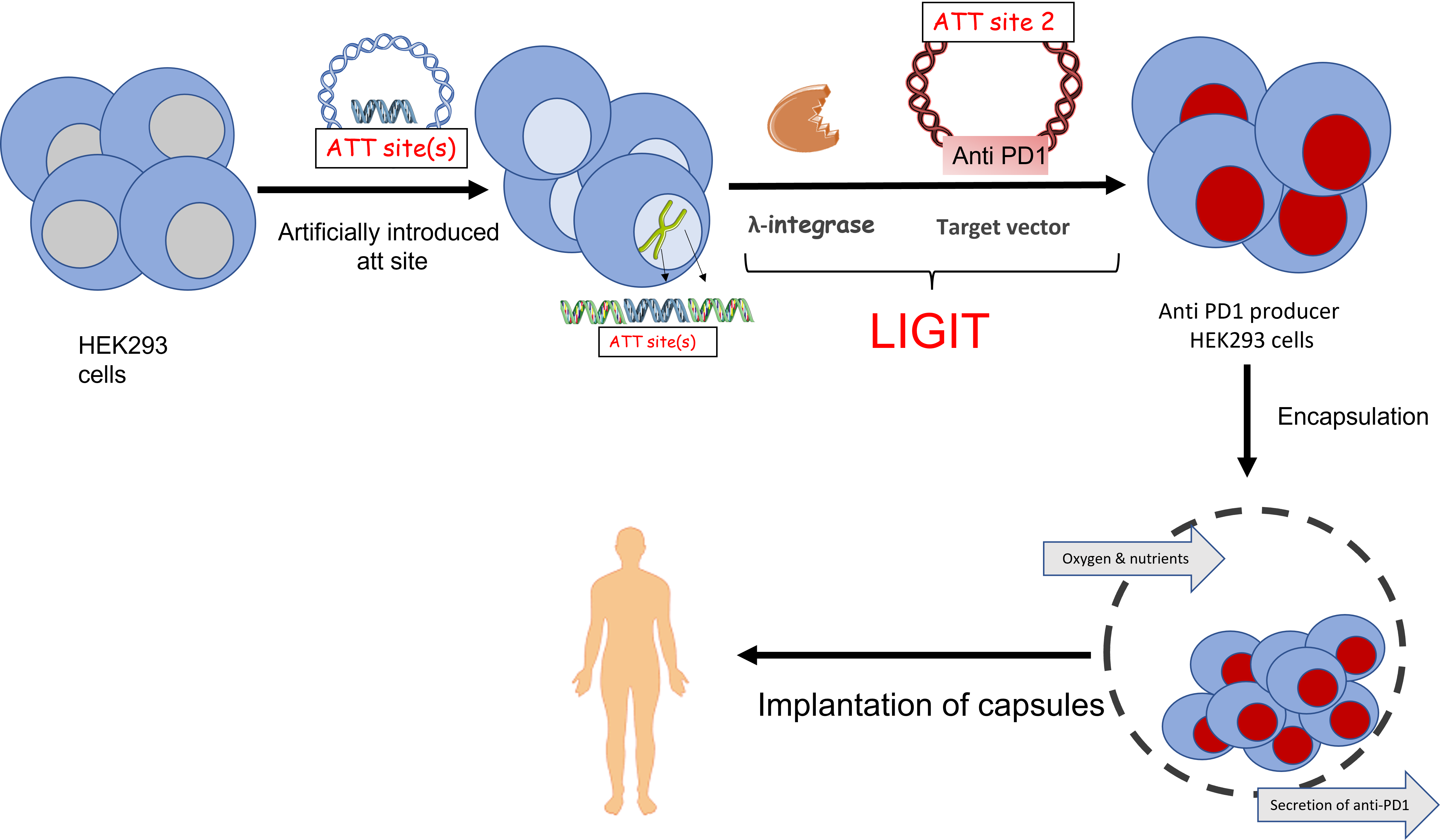
Current focus - Engineering Expi293F (HEK293) with anti-PD1 for cancer immunotherapy
Lambdagen’s strategy involves implantation of immuno-protected, encapsulated producer cell lines to achieve long-term therapeutic effects in patients. In this context, LIGIT has been utilized to target the artificial landing pad with light and heavy chains of PD1 antibodies for stable integration. Currently, we are at the stage of testing our encapsulated cells for in vivo studies in cancer mouse models to study tumor behavior, fibrosis, viability and level of production of antibodies.
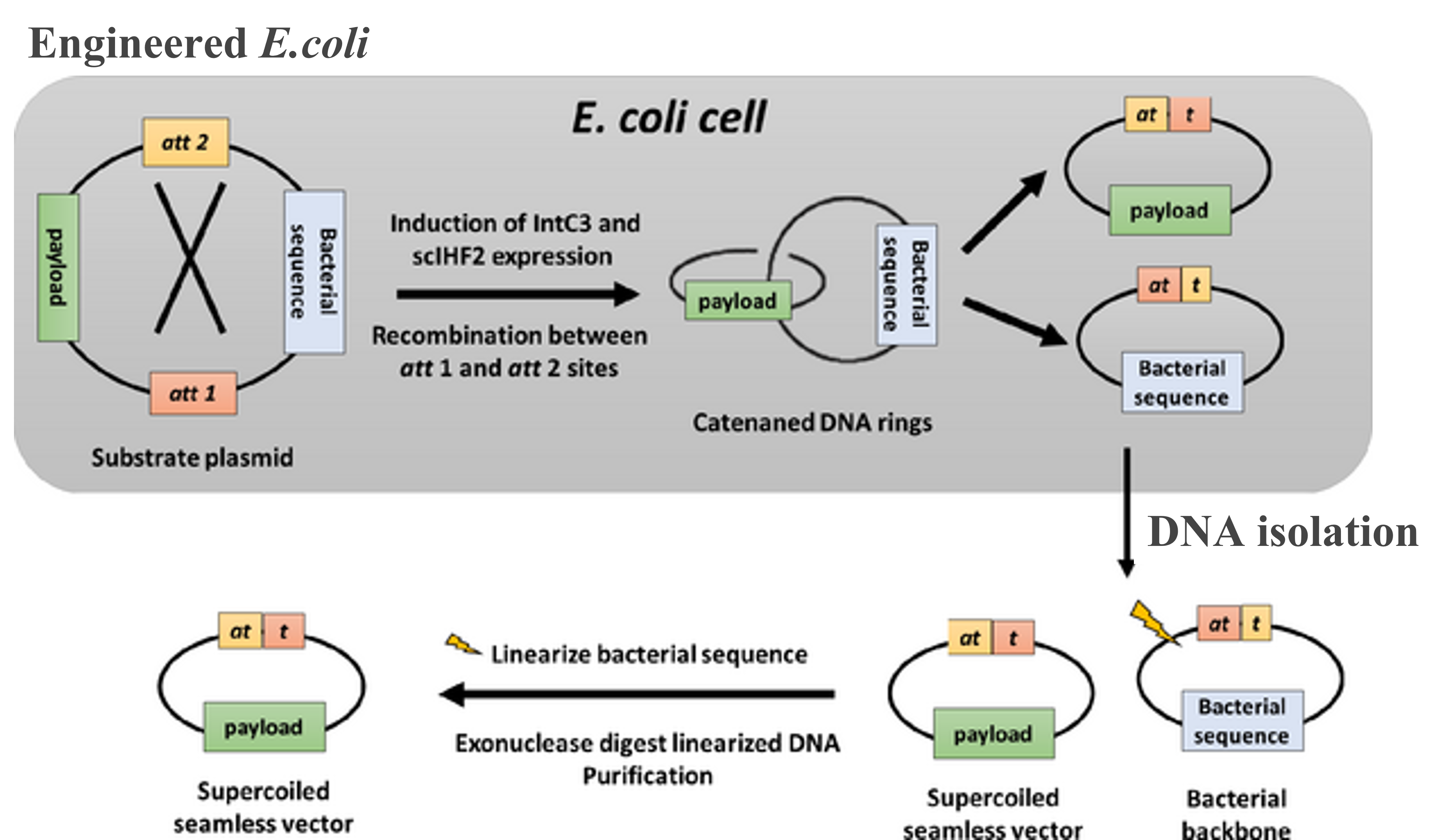
PLATFORM 3: Seamless vector production using engineered Escherichia coli (E. coli)
A novel E. coli strain carrying a tightly regulated gene cassette for the expression of the lambda integrase variant has been generated to efficiently recombine plasmids inside E. coli to yield seamless vectors ranging in size between 500 bp and >10 kb. The DNA is then isolated and cleaved with restriction enzymes together with the highly active phage T5 exonuclease ultimately resulted in high yields of pure negatively supercoiled seamless vectors.
Products & Services
Customized Cell line Engineering
Cell line engineering on the basis of LIGIT with large transgene constructs >10kb
*Validated in 8 different human cell lines including hESCs
Small and Large-scale Seamless Vector (Mini Circles) Production
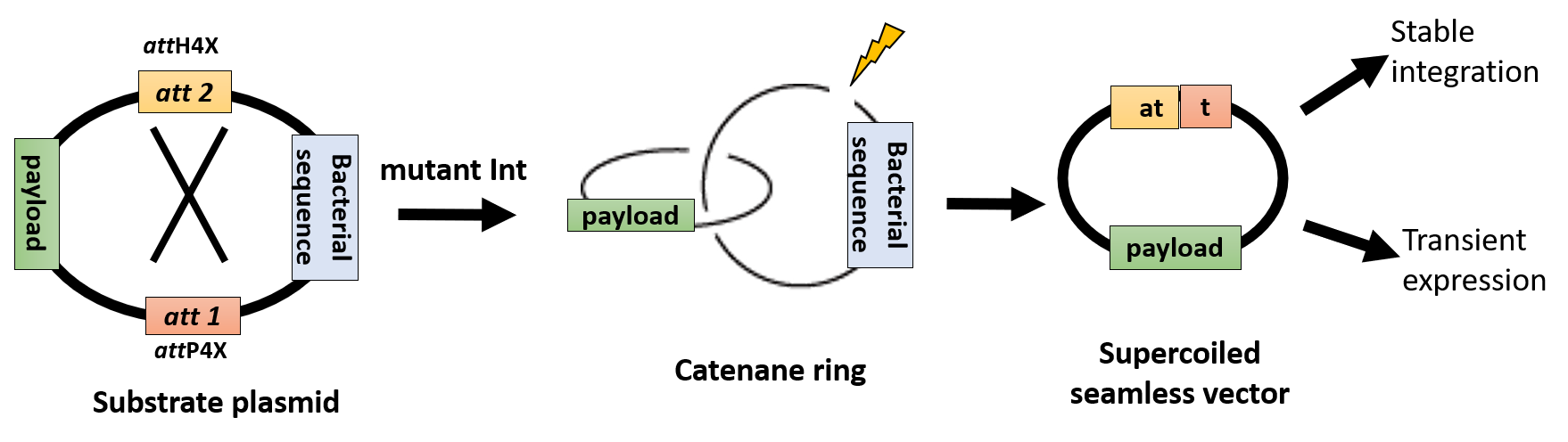
Small- and large-scale seamless vector/DNA minicircle production with novel functional attributes: We offer a versatile production pipeline that can yield large amounts of pure seamless supercoiled vectors ranging in size between < 0.5 kb and>13 kb for various applications. Our seamless vectors carry novel attributes for downstream targeted genome insertion using LIGIT in various species (e.g. human, microalgae) as well as improved transient transgene expression relevant, e.g., for DNA vaccine development.
We offer both in-house seamless vector production and licensing of the production pipeline.
Genome engineering of marine micro-algal cell bioreactors
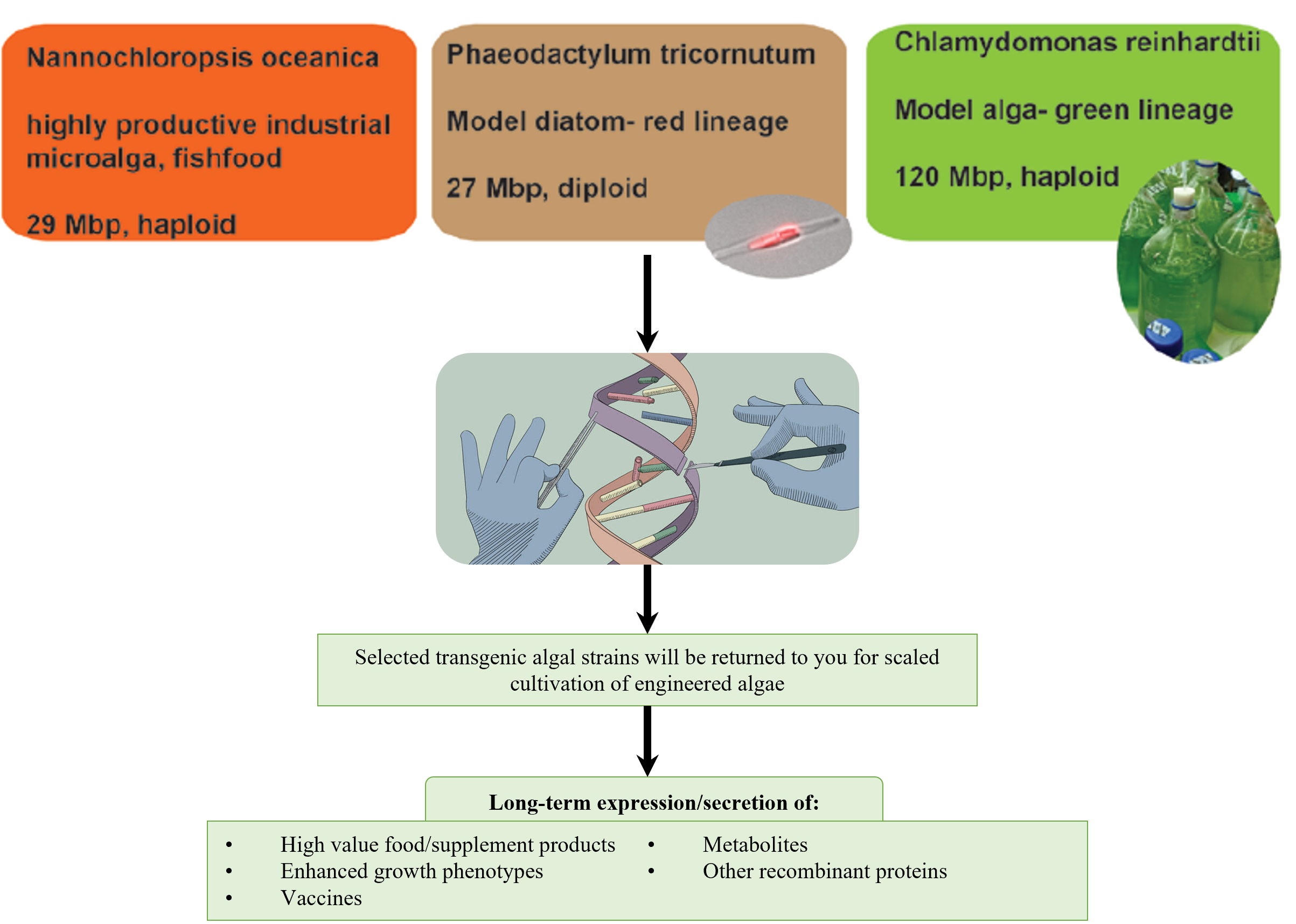
Our propriety integrase platform can help achieve safe and precise genetic engineering in marine microalgae for the controlled and stable expression of recombinant proteins of your choice from artificial genomic docking sites.
Commercial Cell Lines
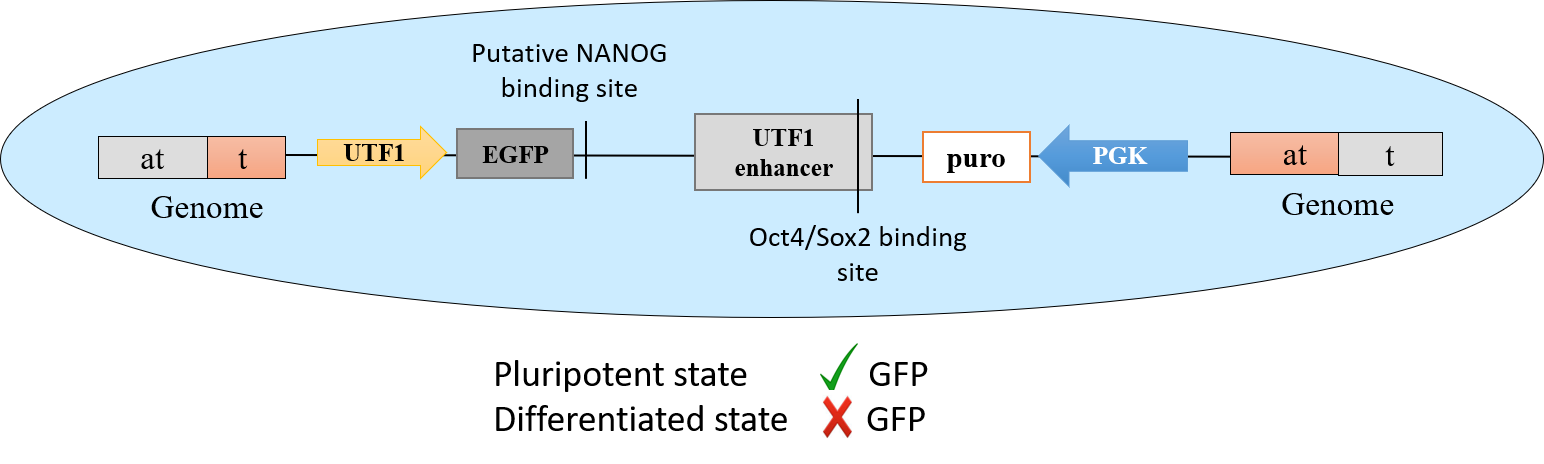
A stable transgenic female hES cell line expressing an Undifferentiated Transcription Factor 1 (UTF1) gene-based pluripotency reporter cassette. UTF1 expression is widely known as one of the most reliable indicator of the pluripotent and even totipotent state in human and mouse cells. We have capitalized on this unique feature and generated this versatile hESC reporter line for various downstream applications.
The selection cassette is constitutively expressed from the targeted genomic integration site on human chromosome #1.
- Drug screening (Hotra et al., 2020, Chaudhari et al., 2020)
- Differentiation studies (Tan et al., 2007, Chandra et al., 2016)
Applications
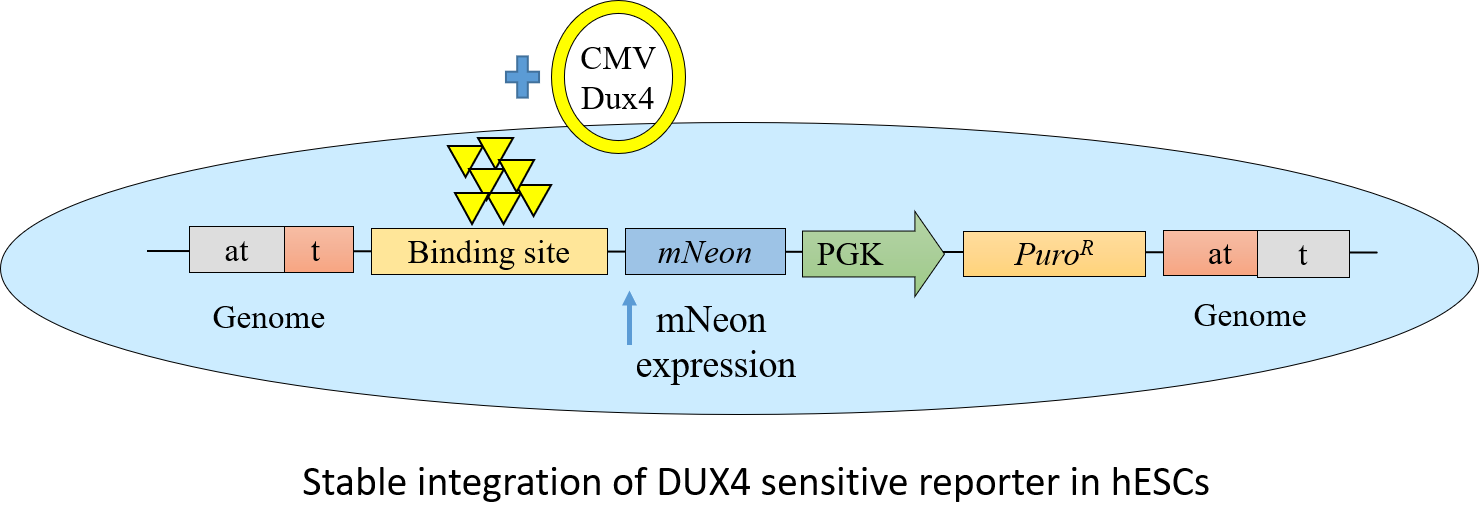
A stem cell-based reporter system which can easily be adapted for high-throughput compound screening for FSHD disease. A Dux4 target gene reporter was developed that responds to the presence of functional Dux4. Upon binding of Dux4 to the array of target sites in the reporter, fluorescent protein mNeon will be expressed. Drug screening can be performed within a 24-48 hours time window to identify molecules that either interfere with or enhance transcriptional reporter activation by Dux4.
- Drug screening (Chaudhari et al., 2020)
Applications
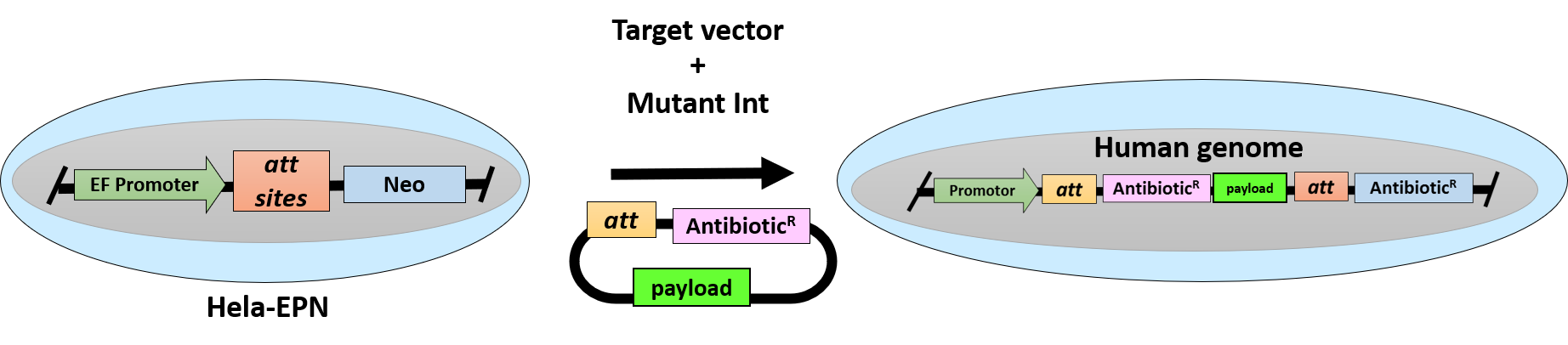
A Hela cell line carrying a single copy of an Artificial Docking site that can be targeted with high precision and efficiency with single or multigene constructs to allow for sustained and highly homogeneous transgene expression without selection pressure. An ideal platform for the screening of mutant protein libraries or the reliable comparison of mutant phenotypes.
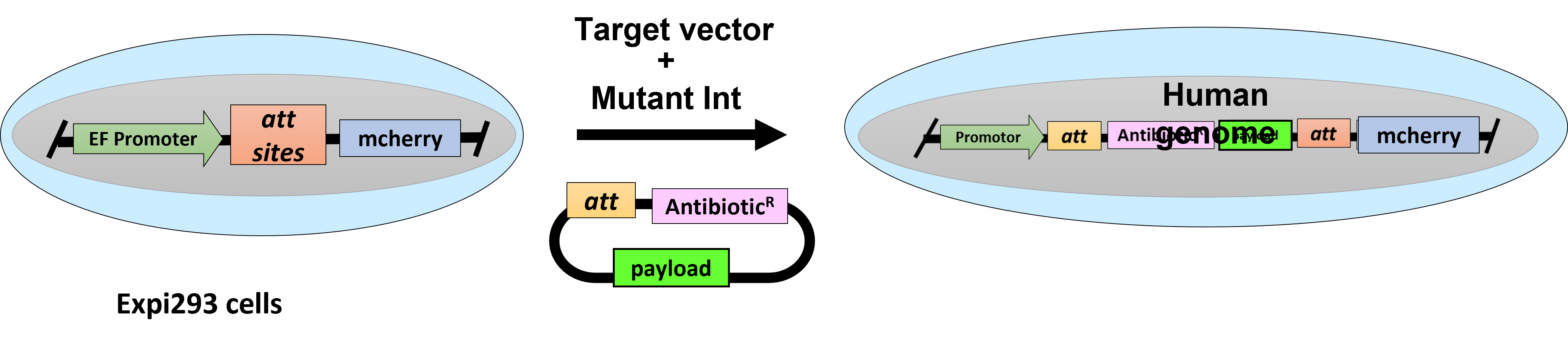
An Expi293 (HEK293) cell line carrying a single copy of an artificial Docking site that can be targeted with high precision and efficiency with single or multigene constructs to allow for sustained and highly homogeneous transgene expression without selection pressure. An ideal platform for a) Screening of mutant protein libraries or the reliable comparison of mutant phenotypes. b) Production of therapeutic proteins
Partners, Support and Funding
Our Pipeline
A robust and differentiated pipeline, leveraging state-of-the-art science to create medicines for serious and rare illness.
| Project | Lead Generation | Discovery | Preclinical | IND | Clinical Trials |
|---|---|---|---|---|---|
| iPSC derived NK cells for Cancer Immunotherapies |
Patents and Publications
| Patent Description | Year of filing | Owner | Inventors |
|---|---|---|---|
| A versatile landing pad platform for transgenesis in human cells using enhanced λ integrases | 2022 | NTU & LambdaGen | Dr Asim Siddiqui, Prof. Peter Droge, Dr Harshyaa Makhija |
| Sequence-specific transgenesis at a novel human genomic safe harbor site with enhanced phage lambda integrase | 2022 | NTU & A*STAR | Dr Asim Siddiqui, Prof. Peter Droge, Dr Harshyaa Makhija and 3 others |
| Multi-scale production of multi-purpose seamless DNA vectors by regulated expression of enhanced λ integrase in Escherichia coli. | 2021 | NTU | Prof. Peter Droge and 2 others |
| DNA Mini-circles for targeted human genome editing | 2017 (Granted) | NTU | Prof. Peter Droge, Dr. Harshyaa Makhija |
| Mutants of the Bacteriophage Lambda Integrase | 2014 (Granted) | A*STAR | Prof. Peter Droge, Dr. Harshyaa Makhija and 6 others |
| Sequence- specific DNA recombination in eukaryotic cells | 2005 (Granted) | Prof. Peter Droge | Prof. Peter Droge |
- Roy S, Peter S, Dröge P. Versatile seamless DNA vector production in E. coli using enhanced phage lambda integrase. PLoS One. 2022 Sep 23;17(9):e0270173
- Chaudhari N, Rickard AM, Roy S, Dröge P, Makhija H. A non-viral genome editing platform for site-specific insertion of large transgenes. Stem Cell Res Ther. 2020 Sep 3;11(1):380
- Hotra A, Ragunathan P, Ng PS, Seankongsuk P, Harikishore A, Sarathy JP, Saw WG, Lakshmanan U, Sae-Lao P, Kalia NP, Shin J, Kalyanasundaram R, Anbarasu S, Parthasarathy K, Pradeep CN, Makhija H, Dröge P, Poulsen A, Tan JHL, Pethe K, Dick T, Bates RW, Grüber G. Discovery of a Novel Mycobacterial F-ATP Synthase Inhibitor and its Potency in Combination with Diarylquinolines. Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13295-13304
- Makhija H, Roy S, Hoon S, Ghadessy FJ, Wong D, Jaiswal R, Campana D, Dröge P. A novel λ integrase-mediated seamless vector transgenesis platform for therapeutic protein expression. Nucleic Acids Res. 2018 Jun 11
- Vijaya Chandra SH, Makhija H, Peter S, Myint Wai CM, Li J, Zhu J, Ren Z, D'Alcontres MS, Siau JW, Chee S, Ghadessy FJ, Dröge P. Conservative site-specific and single-copy transgenesis in human LINE-1 elements. Nucleic Acids Res. 2016 Apr 7;44(6):e55
- Siau JW, Chee S, Makhija H, Wai CM, Chandra SH, Peter S, Dröge P, Ghadessy FJ. Directed evolution of λ integrase activity and specificity by genetic derepression. Protein Eng Des Sel. 2015 Jul;28(7):211-20
Team
Founders

Dr. Harshyaa Makhija
Co-founder and CEO
Prof. Peter Droge
Chief Scientific Advisor
Uday Deshpande
Chief Business Development & Strategy AdvisorAdvisory Board

Prof. Charles Cooney
Scientific and Business Advisor
Dr. Howard Califano
Scientific, IP and Legal AdvisorScientific Collaborators

Prof. Sanjeev Gupta
Albert Einstein College of Medicine, NY (US)
Prof. Francesco Dazzi
King’s College (London)
Prof. Farzin Farzaneh
King’s College (London)
Prof. Dario Campana
National University of Singapore (Singapore)
Dr. Farid Ghadessy
p53 Lab, A*STAR (Singapore)Career
Internship Opportunities
Business intern(s)
LambdaGen (based on a leading edge genome insertion platform technology) is looking for smart, motivated and aspiring candidates who are currently pursuing graduate school or undergrad or IB/A levels to apply for an internship with us. It is a unique opportunity for candidates to build their skills, experience and profile to advance their career in the field of Biotech, Medical profession, Business development and Investment Banking.
Requirements:
- Ambitious and self-driven individual with high expectations of his/her personal growth and development.
- Motivated individual with ‘Can do’ attitude.
- Keen to learn.
- Self-Starter.
- Excellent verbal and written communication skills.
How apply
Please send your resume and cover letter at LambdaGen Therapeutics or write to info@lambdagentherapeutics.com.
Only shortlisted candidates will be notified.
Job openings
Research Scientist
We are seeking an experienced Research Scientist to develop chimeric antigen receptor (CAR) Natural Killer cell-based cancer immunotherapies using proprietary lambda-integrase genome engineering technology. The ideal candidate will assist in the design of targeted iPSC-derived NK cell therapies, provide subject matter expertise for the development of allogeneic cell therapies targeting hematopoietic malignancies, and guide the evaluation of candidate cellular therapies from proof of concept through pre-clinical studies.
Requirements:
- Ph.D. in cellular and molecular biology, genetics, immunology or oncology related field.
- Must have prior hands-on experience with standard molecular biology techniques including cloning, PCR, qRT-PCR, Western blots and mammalian cell culture.
- Strong technical background with gene editing and embryonic stem cell culture.
- Prior experience in developing CAR-based immunotherapies is highly desirable.
- Experience with multiparametric flow cytometry.
- In vivo mouse experimentation with cancer models is a plus.
- Plan and execute experiments and analyze data independently and contribute to team science.
- Strong organizational, data management, research report and manuscript preparation.
How apply
Please send your resume and cover letter at LambdaGen Therapeutics or write to info@lambdagentherapeutics.com.
Only shortlisted candidates will be notified.
Join Us
Collaborators/Alliances
We are actively looking for collaborators or partners to be a part of our research and development and product development journey.
Investors
We are constantly looking for investment opportunities to propel our initiatives forward.
Contact Us
Use the form below to drop us an e-mail. Old-fashioned phone calls work too













